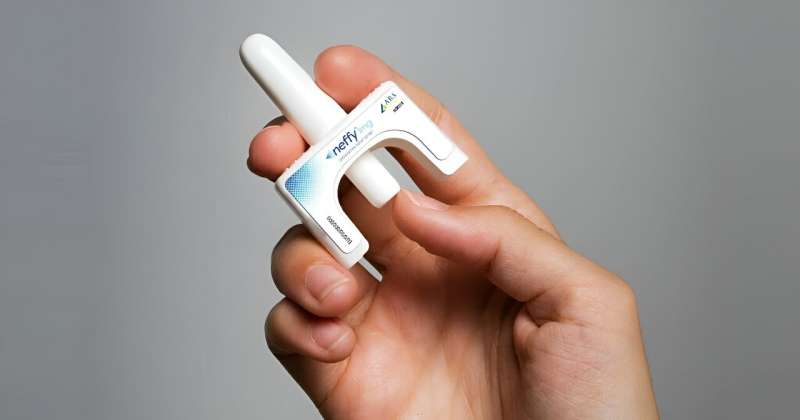
People nervous about administering a rescue shot for anaphylaxis lastly have a brand new different in a nasal spray.
The U.S. Meals and Drug Administration on Friday introduced that it has authorised neffy, the primary non-injected therapy for life-threatening allergic reactions.
The epinephrine nasal spray is to be used by adults and youngsters who weigh greater than 66 kilos, the company stated.
“Anaphylaxis is life-threatening and a few folks, significantly kids, could delay or keep away from therapy because of concern of injections,” Dr. Kelly Stone, affiliate director of the Division of Pulmonology, Allergy and Essential Care within the FDA’s Middle for Drug Analysis and Analysis, stated in an company assertion. “The provision of epinephrine nasal spray could scale back limitations to fast therapy of anaphylaxis. In consequence, neffy offers an necessary therapy possibility and addresses an unmet want.”
People with allergy symptoms can expertise a sudden, scary response to allergens—usually sure meals, drugs or insect stings.
Till now, epinephrine has been the one rescue treatment when such incidents happen, and it is solely been administered by way of a needle.
Neffy, made by ARS Prescribed drugs, is delivered as a twig spritzed into one nostril, the FDA stated. If the primary dose would not ease signs, the company urges giving a second dose (from a brand new dispenser) into the identical nostril. Monitor sufferers intently after epinephrine is used, in case additional therapy, together with emergency medical assist, is required.
“Neffy’s approval is predicated on 4 research in 175 wholesome adults, with out anaphylaxis, that measured the epinephrine concentrations within the blood following administration of neffy or authorised epinephrine injection merchandise,” the FDA stated. “Outcomes from these research confirmed comparable epinephrine blood concentrations between neffy and authorised epinephrine injection merchandise.”
The research additionally confirmed that neffy was efficient in triggering the sorts of will increase in blood strain and coronary heart fee which can be essential to treating anaphylaxis.
Neffy can include uncomfortable side effects. These could embody: throat irritation, tingling nostril, headache, nasal discomfort, feeling jittery, tingling sensation, fatigue, tremor, runny nostril, itchiness contained in the nostril (nasal pruritus), sneezing, belly ache, gum ache, numbness within the mouth, nasal congestion, dizziness, nausea and vomiting.
“Neffy comes with a warning that sure nasal situations, comparable to nasal polyps or a historical past of nasal surgical procedure, could have an effect on absorption of neffy, and sufferers with these situations ought to seek the advice of with a well being care skilled to contemplate use of an injectable epinephrine product,” the FDA added. “Neffy additionally comes with warnings and precautions about use of epinephrine by folks with sure coexisting situations and allergic reactions related to sulfite.”
The brand new approval comes after an skilled advisory panel supported neffy’s use again in Might of 2023. In September, the FDA held again on approval, asking for additional examine.
Extra info:
Study the indicators of anaphylaxis on the Cleveland Clinic.
SOURCE: U.S. Meals and Drug Administration, announcement, Aug. 9, 2024
Copyright © 2024 HealthDay. All rights reserved.
Quotation:
FDA approves first nasal spray to curb anaphylaxis, an alternative choice to injections (2024, August 10)
retrieved 10 August 2024
from https://medicalxpress.com/information/2024-08-fda-nasal-spray-curb-anaphylaxis.html
This doc is topic to copyright. Other than any truthful dealing for the aim of personal examine or analysis, no
half could also be reproduced with out the written permission. The content material is supplied for info functions solely.

















